Does Boron Form Ionic Bonds . Borax ore (known as tincal); it makes stable covalent bonds with other compounds and does not forms ionic bonds. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. You won't get monatomic cations like the metals below it. boron can form ions but there is some fine print. Boron doesn't form ions because the total energy needed to. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. Boron combines with air to form boron trioxide, which acts a protective. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead.
from www.schoolsobservatory.org
You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. You won't get monatomic cations like the metals below it. boron can form ions but there is some fine print. it makes stable covalent bonds with other compounds and does not forms ionic bonds. Borax ore (known as tincal); a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. Boron doesn't form ions because the total energy needed to. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Boron combines with air to form boron trioxide, which acts a protective.
Semiconductors The Schools' Observatory
Does Boron Form Ionic Bonds boron can form ions but there is some fine print. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. Boron combines with air to form boron trioxide, which acts a protective. Boron doesn't form ions because the total energy needed to. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. You won't get monatomic cations like the metals below it. boron can form ions but there is some fine print. it makes stable covalent bonds with other compounds and does not forms ionic bonds. Borax ore (known as tincal); instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that.
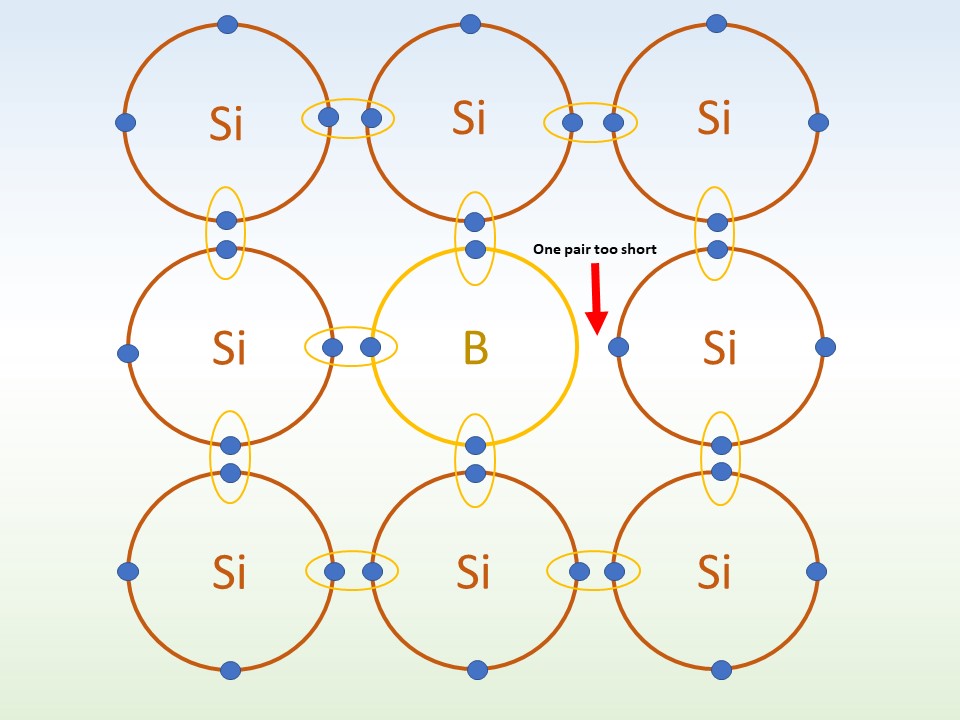
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Does Boron Form Ionic Bonds You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. Boron combines with air to form boron trioxide, which acts a protective. You won't get monatomic cations like the metals below it. Borax ore (known as tincal); it makes stable covalent bonds with other compounds and does not forms ionic bonds. instead of forming a. Does Boron Form Ionic Bonds.
From www.youtube.com
Is BF3 (Boron trifluoride) Ionic or Covalent/Molecular? YouTube Does Boron Form Ionic Bonds a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. boron can form ions but there is some fine print. Boron doesn't form ions because the total energy needed to. Boron combines with air to form boron trioxide, which acts a protective. You might perhaps wonder why. Does Boron Form Ionic Bonds.
From www.youtube.com
B 3+ Electron Configuration (Boron Ion) YouTube Does Boron Form Ionic Bonds Boron combines with air to form boron trioxide, which acts a protective. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. You might perhaps wonder why boron doesn't form ionic bonds. Does Boron Form Ionic Bonds.
From www.nagwa.com
Question Video Understanding How Ammonia Molecules Bond Boron Does Boron Form Ionic Bonds Boron doesn't form ions because the total energy needed to. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Borax ore (known as tincal); You might perhaps wonder why boron doesn't. Does Boron Form Ionic Bonds.
From borates.today
Boron Electron Valence Borates Today Does Boron Form Ionic Bonds a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. Borax ore (known as tincal); boron can form ions but there is some fine print. it makes stable covalent bonds with other compounds and does not forms ionic bonds. You won't get monatomic cations like the. Does Boron Form Ionic Bonds.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Does Boron Form Ionic Bonds a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Boron combines with air to form boron trioxide, which acts a protective. You might perhaps wonder why boron doesn't form ionic bonds. Does Boron Form Ionic Bonds.
From www.slideserve.com
PPT Ionic Bonding and MainGroup Elements PowerPoint Presentation Does Boron Form Ionic Bonds You won't get monatomic cations like the metals below it. boron can form ions but there is some fine print. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Borax ore (known as tincal); it makes stable covalent bonds with other compounds and does not forms ionic bonds. You might perhaps. Does Boron Form Ionic Bonds.
From periodictable.me
How To Find The Electron Configuration For Boron Dynamic Periodic Does Boron Form Ionic Bonds Borax ore (known as tincal); You won't get monatomic cations like the metals below it. Boron doesn't form ions because the total energy needed to. it makes stable covalent bonds with other compounds and does not forms ionic bonds. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. boron can form. Does Boron Form Ionic Bonds.
From schematron.org
Lewis Dot Diagram For Boron Wiring Diagram Pictures Does Boron Form Ionic Bonds boron can form ions but there is some fine print. Borax ore (known as tincal); it makes stable covalent bonds with other compounds and does not forms ionic bonds. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Boron doesn't form ions because the total energy needed to. Boron combines with. Does Boron Form Ionic Bonds.
From littleeagles.edu.vn
Boron Valence Electrons Boron Valency (B) With Dot Diagram Does Boron Form Ionic Bonds it makes stable covalent bonds with other compounds and does not forms ionic bonds. You won't get monatomic cations like the metals below it. Boron combines with air to form boron trioxide, which acts a protective. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Borax ore (known as tincal); You might. Does Boron Form Ionic Bonds.
From www.sciencephoto.com
Boron, atomic structure Stock Image C018/3686 Science Photo Library Does Boron Form Ionic Bonds it makes stable covalent bonds with other compounds and does not forms ionic bonds. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. Boron doesn't form ions because the total energy needed to. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead.. Does Boron Form Ionic Bonds.
From www.examples.com
Boron (B) Definition, Preparation, Properties, Uses, Compounds Does Boron Form Ionic Bonds instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Boron combines with air to form boron trioxide, which acts a protective. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. it makes stable covalent bonds with other compounds and. Does Boron Form Ionic Bonds.
From mavink.com
Boron Model Does Boron Form Ionic Bonds Borax ore (known as tincal); a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. it makes stable covalent bonds with other compounds and does not forms ionic bonds. Boron combines with air to. Does Boron Form Ionic Bonds.
From www.youtube.com
Lewis Dot Structure for BoronHow do you draw the Lewis Dot structure Does Boron Form Ionic Bonds Boron combines with air to form boron trioxide, which acts a protective. it makes stable covalent bonds with other compounds and does not forms ionic bonds. a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. You might perhaps wonder why boron doesn't form ionic bonds with. Does Boron Form Ionic Bonds.
From www.pinterest.com
Coordinate Covalent Bond Dative Bond Boron Atom, Ionic Compound Does Boron Form Ionic Bonds You won't get monatomic cations like the metals below it. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. Borax ore (known as tincal); instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. boron can form ions but there is some fine print. Boron combines with air to. Does Boron Form Ionic Bonds.
From www.science.org
A BoronBoron Triple Bond Science Does Boron Form Ionic Bonds a bond is ionic if the electronegativity difference between the atoms is great enough that one atom could pull an electron. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. instead of forming a metallic lattice with delocalized valence electrons, boron forms unique aggregates that. Borax ore (known as tincal); it makes stable. Does Boron Form Ionic Bonds.
From www.nagwa.com
Question Video Explaining the Covalent Bonding in Borane Nagwa Does Boron Form Ionic Bonds Borax ore (known as tincal); You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. boron can form ions but there is some fine print. it makes stable covalent bonds with other compounds and does not forms ionic bonds. You won't get monatomic cations like the metals below it. Boron combines with air to form. Does Boron Form Ionic Bonds.
From www.britannica.com
boron Properties, Uses, & Facts Britannica Does Boron Form Ionic Bonds boron can form ions but there is some fine print. it makes stable covalent bonds with other compounds and does not forms ionic bonds. Boron doesn't form ions because the total energy needed to. You might perhaps wonder why boron doesn't form ionic bonds with fluorine instead. You won't get monatomic cations like the metals below it. Boron. Does Boron Form Ionic Bonds.